pH-Dependent Spin State Population and ¹⁹F NMR Chemical Shift Via Remote Ligand Protonation in An Iron(II) Complex
An FeII complex that features a pH-dependent spin state population demonstrates potential as a 19F chemical shift-based pH sensor.
The development of transition metal-based molecules and materials that can be switched between low-spin and high-spin electronic states has constituted a highly active area of research over the past several decades. Indeed, the magnetic bistability of such spin-crossover compounds make them potential candidates for molecular switches and chemical sensors, as the spin transition can be controlled by a number of external stimuli, such as temperature, pressure, and light.
Recently, researchers have begun to explore the potential for spin-switchable molecules as bioresponsive probes for temperature, anions, and enzyme activity. In addition, given the relationship between tissue acidosis and diseases, including cancer and ischemia, a compound that undergoes a spin state transition as a function of pH could serve as a valuable tool for pH sensing. Nevertheless, pH-induced spin state switching is rare, and compounds that exhibit such behavior are unsuitable for most biological sensing applications due to pKa values far from biological pH or poor stability in water.
One approach toward biological pH sensing is to employ pH-induced changes in the chemical shift of 19F resonances, typically caused by an interconversion between species of different protonation states. Here, the use of 19F magnetic resonance spectroscopy (MRS) offers key advantages over the more commonly employed 1H MRS, most notably the absence of endogenous fluorine in living systems. Furthermore, the chemical shift of the 19F nucleus is highly sensitive to its local environment, such that small chemical changes can lead to drastic changes in chemical shift. Indeed, diamagnetic 19F MRS pH probes with pKa values near 7 have been developed for in vivo applications, where a variation in 19F chemical shift of 12 ppm was observed between the protonated and deprotonated forms. In addition, the sensitivity of 19F MRS probes can be further improved by incorporating paramagnetic metal ions, as the difference in chemical shift between the protonated and deprotonated forms is amplified by the presence of contact (through-bond) and dipolar (through-space) contributions to the chemical shift.
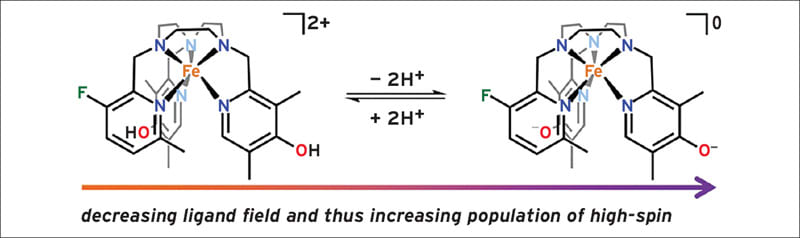
It has been previously demonstrated that spin-crossover FeII complexes can facilitate chemical shift-based MR thermometry. Here, since both the contact and dipolar contributions to the paramagnetic chemical shift scale with S(S + 1), where S is the electronic spin state, thermally-induced spin-crossover from an S =0 ground state to an S = 2 excited state afforded a dramatic increase in chemical shift with temperature. Building on these results, an attempt was made to develop a spin-crossover FeII complex that undergoes a deprotonation-induced spin state change near biological pH for 19F chemical shift-based pH sensing. Herein are reported the synthesis and characterization of an FeII complex that features a new asymmetric 1,4,7-triazacyclononane (TACN) ligand appended with two 4-hydroxylpyridine donors, and it was demonstrated that pH-induced spin state switching can engender highly sensitive 19F MRS pH probes.
In order to develop an 19F MR probe that undergoes a pH-induced spin state transition, the decision was made to design a ligand that (1) forms a water-soluble complex with FeII, (2) features an 19F reporter group, and (3) affords a ligand field that changes dramatically with pH. Toward this end, 4-hydroxy- 3,5-dimethyl-2-pyridyl groups were selected as the pH sensing moieties, because the ability of 4-hydroxylpyridines to engender significant changes in the electronic structure of transition metal compounds upon protonation or deprotonation has been demonstrated in V-, Fe-, Co-, Ni-, Ru-, Pt-, Re-, and Ir-based compounds. It has thus been hypothesized that incorporating hydroxylpyridine groups into a spin-crossover complex could create a compound with a pH-sensitive spin state population (see Figure).
This work was done by Alexandra I. Gaudette, Agnes E. Thorarinsdottir, and T. David Harris of Northwestern University for the Air Force Research Laboratory. AFRL-0258
This Brief includes a Technical Support Package (TSP).

pH-Dependent Spin State Population and 19F NMR Chemical Shift Via Remote Ligand Protonation in An Iron(II) Complex
(reference AFRL-0258) is currently available for download from the TSP library.
Don't have an account?
Overview
The document titled "pH-Dependent Spin State Population and 19F NMR Chemical Shift via Remote Ligand Protonation in an Iron(II) Complex" presents research conducted by Alexandra I. Gaudette, Agnes E. Thorarinsdottir, and T. David Harris from Northwestern University. Published in the journal Chemical Communications, this interim report discusses the behavior of an iron(II) complex that exhibits a pH-dependent spin state population due to variable ligand protonation.
The study highlights the significance of pH in influencing the spin state of the iron(II) complex, which is crucial for understanding its chemical properties and potential applications. The researchers observed that as the pH changes, the spin state population shifts, leading to variations in the 19F NMR chemical shift. Specifically, the sensitivity of the chemical shift was measured at 13.9 ppm per pH unit at 37°C, indicating the potential of this complex as a pH sensor.
The findings are supported by variable-pH behavior observed in UV-Visible analysis, confirming the relationship between pH and spin state population. The study provides quantitative data, showing that the volume change associated with the spin state transition varies with pH, with values ranging from 2.20 cm³ K mol⁻¹ at pH 4.74 (73% high-spin) to 2.65 cm³ K mol⁻¹ at pH 7.82 (88% high-spin).
The document also acknowledges funding support from the Alfred P. Sloan Foundation and the Leifur Eiriksson Foundation, and it declares no conflicts of interest. The authors emphasize that the views and conclusions presented do not necessarily represent the official policies of the Air Force Research Laboratory or the U.S. Government.
Overall, this research contributes to the understanding of spin crossover phenomena in transition metal complexes and opens avenues for developing chemical sensors based on NMR techniques. The implications of this work extend to various fields, including materials science and analytical chemistry, where pH-sensitive materials can play a critical role in sensing and detection applications.
Top Stories
NewsRF & Microwave Electronics
![]() Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
INSIDERAerospace
![]() A Next Generation Helmet System for Navy Pilots
A Next Generation Helmet System for Navy Pilots
INSIDERDesign
![]() New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
INSIDERMaterials
![]() How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
NewsPower
![]() Ford Announces 48-Volt Architecture for Future Electric Truck
Ford Announces 48-Volt Architecture for Future Electric Truck
ArticlesAR/AI
Webcasts
Electronics & Computers
![]() Cooling a New Generation of Aerospace and Defense Embedded...
Cooling a New Generation of Aerospace and Defense Embedded...
Automotive
![]() Battery Abuse Testing: Pushing to Failure
Battery Abuse Testing: Pushing to Failure
Power
![]() A FREE Two-Day Event Dedicated to Connected Mobility
A FREE Two-Day Event Dedicated to Connected Mobility
Unmanned Systems
![]() Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Automotive
![]() Advantages of Smart Power Distribution Unit Design for Automotive &...
Advantages of Smart Power Distribution Unit Design for Automotive &...
Energy
![]() Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance
Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance