Hybrid Membrane Doubles the Lifetime of Rechargeable Batteries
The membrane prevents dendrite formation, at least doubling the lifetime of a lithium-metal battery.
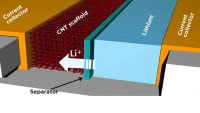
The energy density of traditional lithium-ion batteries is approaching a saturation point that cannot meet the demands of the future; for example, in electric vehicles. Lithium-metal batteries can provide double the energy per unit weight when compared to lithium-ion batteries. The biggest challenge, however, is the formation of lithium dendrites — small, needle-like structures — over the lithium-metal anode. These dendrites often continue to grow until they pierce the separator membrane, causing the battery to short-circuit and ultimately destroy it. Researchers have developed a solution to prevent dendrite formation and thus at least double the lifetime of a lithium-metal battery. During the charge transfer process, lithium ions move back and forth between the anode and the cathode. Whenever they pick up an electron, they deposit lithium atoms, which accumulate on the anode. A crystalline surface is formed, which grows three-dimensionally where the atoms accumulate, creating the dendrites. The pores of the separator membrane influence the nucleation of dendrites. If ion transport is more homogeneous, dendrite nucleation can be avoided.
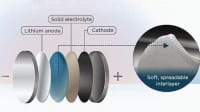
The researchers applied an extremely thin, two-dimensional membrane — called a Hybrid Separator Membane — made of carbon to the separator, with the pores having a diameter of less than one nanometer. These tiny openings are smaller than the critical nucleus size and thus prevent the nucleation that leads to the formation of dendrites. Instead of forming dendritic structures, the lithium is deposited on the anode as a smooth film. There is no risk of the separator membrane being damaged by this and the functionality of the battery is not affected.

To test the method, the researchers repeatedly recharged test batteries fitted with the membrane. Even after hundreds of charging and discharging cycles, no dendritic growth was detected. The key innovation is the stabilizing electrode/electrolyte interface with an ultra-thin membrane that does not alter the current battery manufacturing process. Interface stability is also key to enhancing the performance and safety of an electrochemical system.
High-energy-density batteries extend the driving range of electric vehicle (EVs) for the same weight/volume of the battery that a modern EV possesses and make portable electronic devices last longer in a single charge.
The next step is to see how the application of the two-dimensional membrane can be integrated into the manufacturing process. The researchers also want to apply the idea to other types of batteries.
For more information, contact Professor Dr. Andrey Turchanin at
Top Stories
INSIDERManufacturing & Prototyping
![]() How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
NewsAutomotive
![]() Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
INSIDERAerospace
![]() A Next Generation Helmet System for Navy Pilots
A Next Generation Helmet System for Navy Pilots
INSIDERDesign
![]() New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
ArticlesAR/AI
![]() CES 2026: Bosch is Ready to Bring AI to Your (Likely ICE-powered) Vehicle
CES 2026: Bosch is Ready to Bring AI to Your (Likely ICE-powered) Vehicle
Road ReadyDesign
Webcasts
Semiconductors & ICs
![]() Advantages of Smart Power Distribution Unit Design for Automotive...
Advantages of Smart Power Distribution Unit Design for Automotive...
Unmanned Systems
![]() Quiet, Please: NVH Improvement Opportunities in the Early Design...
Quiet, Please: NVH Improvement Opportunities in the Early Design...
Electronics & Computers
![]() Cooling a New Generation of Aerospace and Defense Embedded...
Cooling a New Generation of Aerospace and Defense Embedded...
Power
![]() Battery Abuse Testing: Pushing to Failure
Battery Abuse Testing: Pushing to Failure
AR/AI
![]() A FREE Two-Day Event Dedicated to Connected Mobility
A FREE Two-Day Event Dedicated to Connected Mobility