Researchers Develop Efficient Lithium Extraction Method, Setting Stage for Sustainable EV Battery Supply Chains
Solid-state electrolyte membranes revolutionize lithium harvesting with near-perfect selectivity.
In the race to meet the growing global demand for lithium, a team of researchers from Rice University’s Elimelech lab has developed a breakthrough lithium extraction method that could reshape the industry.

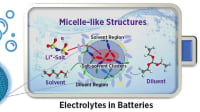
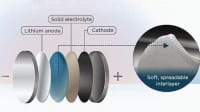
In their study published in Science Advances, the researchers demonstrated near-perfect lithium selectivity by repurposing solid-state electrolytes (SSEs) as membrane materials for aqueous lithium extraction. While originally designed for the rapid conduction of lithium ions in solid-state batteries — where there are no other ions or liquid solvents — the highly ordered and confined structure of SSEs was found to enable unprecedented separation of both ions and water in aqueous mixtures.
This discovery presents a potential breakthrough in sustainable resource recovery, reducing reliance on traditional mining and extraction techniques that are both time-consuming and environmentally damaging.
“The challenge is not just about increasing lithium production but about doing so in a way that is both sustainable and economically viable,” said corresponding author Menachem Elimelech, the Nancy and Clint Carlson Professor of Civil and Environmental Engineering.
To make lithium extraction more environmentally sustainable, researchers have been exploring direct lithium extraction technologies that recover lithium from unconventional sources such as oil- and gas-produced water, industrial wastewater, and geothermal brines. These methods, however, have struggled with ion selectivity, particularly when trying to separate lithium from other ions of similar size or charge like magnesium and sodium.
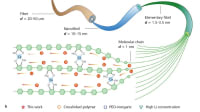
The novel approach developed by Elimelech and his team hinges on a fundamental difference between SSEs and conventional nanoporous membranes. Whereas traditional membranes rely on hydrated nanoscale pores to transport ions, SSEs shuttle lithium ions through an anhydrous hopping mechanism within a highly ordered crystalline lattice.
“This means that lithium ions can migrate through the membrane while other competing ions, and even water, are effectively blocked,” said first author Sohum Patel, who is now a postdoctoral researcher at the Massachusetts Institute of Technology. “The extreme selectivity offered by our SSE-based approach makes it a highly efficient method for lithium harvesting, as energy is only expended for moving the desired lithium ions across the membrane.”
The research team, which also includes Arpita Iddya, Weiyi Pan, and Jianhao Qian — postdoctoral researchers in Elimelech’s lab at Rice — tested this phenomenon using an electrodialysis setup, where an applied electric field drove lithium ions across the membrane. The results were striking. Even at high concentrations of competing ions, the SSE consistently demonstrated near-perfect lithium selectivity with no detectable competing ions in the product stream — something conventional membrane technologies have been unable to achieve.
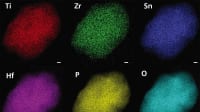
Using a combination of computational and experimental techniques, the team investigated why the SSEs exhibited such remarkable lithium-ion selectivity. Their findings revealed that the rigid and tightly packed crystalline lattice of the SSE prevented water molecules and larger ions like sodium from passing through the membrane structure. Magnesium ions, which have a different charge than lithium ions, were also found to be incompatible with the crystal structure and were thus rejected.
“The lattice acts as a molecular sieve, allowing only lithium ions to pass through,” said Elimelech. “This combination of highly precise size and charge exclusion is what makes the SSE membrane so unique.”
The researchers noted that while competing ions did not penetrate the SSE, their presence in the feed solution reduced lithium flux by blocking available surface sites for ion exchange, a challenge they believe can be addressed through further material engineering.
“By integrating SSEs into electrodialysis systems, we could enable direct lithium extraction from a range of aqueous sources, reducing the need for large evaporation ponds and chemical-intensive purification steps,” said Patel. “This could significantly lower the environmental footprint of lithium production while making the process more efficient.”
The findings also suggest broader applications beyond lithium for SSEs in ion-selective separations. “The mechanisms of ion selectivity in SSEs could inspire the development of similar membranes for extracting other critical elements from water sources,” said Elimelech. “This could open the door to a new class of membrane materials for resource recovery.”
For more information, contact Alexandra Becker at
Top Stories
INSIDERManufacturing & Prototyping
![]() How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
NewsAutomotive
![]() Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
INSIDERAerospace
![]() A Next Generation Helmet System for Navy Pilots
A Next Generation Helmet System for Navy Pilots
INSIDERDesign
![]() New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
ArticlesAR/AI
![]() CES 2026: Bosch is Ready to Bring AI to Your (Likely ICE-powered) Vehicle
CES 2026: Bosch is Ready to Bring AI to Your (Likely ICE-powered) Vehicle
Road ReadyDesign
Webcasts
Semiconductors & ICs
![]() Advantages of Smart Power Distribution Unit Design for Automotive...
Advantages of Smart Power Distribution Unit Design for Automotive...
Unmanned Systems
![]() Quiet, Please: NVH Improvement Opportunities in the Early Design...
Quiet, Please: NVH Improvement Opportunities in the Early Design...
Electronics & Computers
![]() Cooling a New Generation of Aerospace and Defense Embedded...
Cooling a New Generation of Aerospace and Defense Embedded...
Power
![]() Battery Abuse Testing: Pushing to Failure
Battery Abuse Testing: Pushing to Failure
AR/AI
![]() A FREE Two-Day Event Dedicated to Connected Mobility
A FREE Two-Day Event Dedicated to Connected Mobility