Folded or Cut, This Lithium-Sulfur Battery Keeps Going
A lithium-sulfur battery features an improved iron sulfide cathode. One prototype remains highly stable over 300 charge-discharge cycles, and another provides power even after being folded or cut.
Most rechargeable batteries that power portable devices, such as toys, handheld vacuums, and e-bikes, use lithium-ion technology. But these batteries can have short lifetimes and may catch fire when damaged. To address stability and safety issues, researchers reporting in ACS Energy Letters have designed a lithium-sulfur (Li-S) battery that features an improved iron sulfide cathode. One prototype remains highly stable over 300 charge-discharge cycles, and another provides power even after being folded or cut.
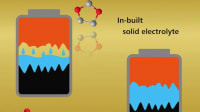
Sulfur has been suggested as a material for lithium-ion batteries because of its low cost and potential to hold more energy than lithium-metal oxides and other materials used in traditional ion-based versions. To make Li-S batteries stable at high temperatures, researchers have previously proposed using a carbonate-based electrolyte to separate the two electrodes (an iron sulfide cathode and a lithium metal-containing anode). However, as the sulfide in the cathode dissolves into the electrolyte, it forms an impenetrable precipitate, causing the cell to quickly lose capacity. Liping Wang and colleagues wondered if they could add a layer between the cathode and electrolyte to reduce this corrosion without reducing functionality and rechargeability.
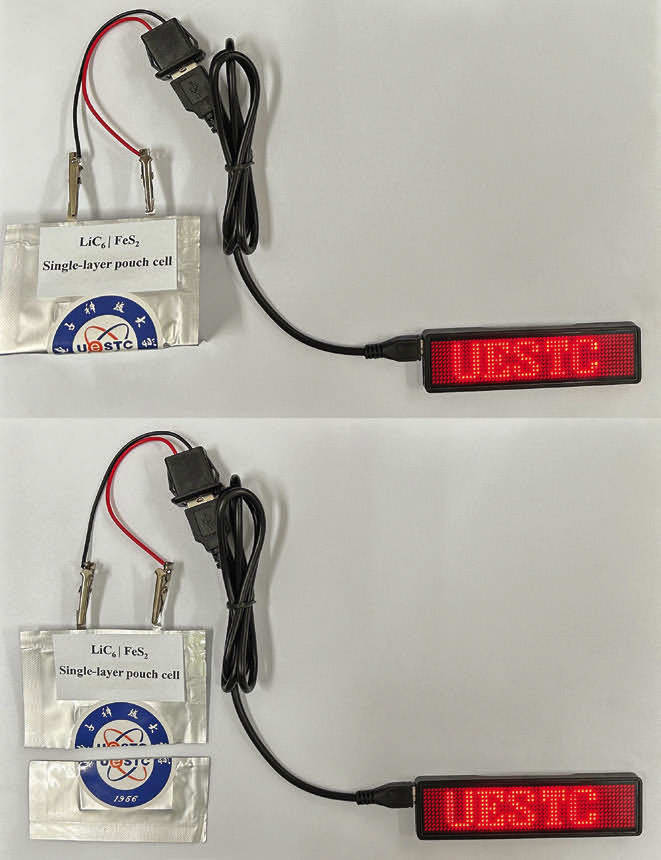
The team coated iron sulfide cathodes in different polymers and found in initial electrochemical performance tests that polyacrylic acid (PAA) performed best, retaining the electrode’s discharge capacity after 300 charge-discharge cycles. Next, the researchers incorporated a PAA-coated iron sulfide cathode into a prototype battery design, which also included a carbonate-based electrolyte, a lithium metal foil as an ion source, and a graphite-based anode. They produced and then tested both pouch cell and coin cell battery prototypes.
After more than 100 charge-discharge cycles, Wang and colleagues observed no substantial capacity decay in the pouch cell. Additional experiments showed that the pouch cell still worked after being folded and cut in half. The coin cell retained 72 percent of its capacity after 300 charge-discharge cycles. They next applied the polymer coating to cathodes made from other metals, creating lithium-molybdenum and lithium-vanadium batteries. These cells also had stable capacity over 300 charge-discharge cycles. Overall, the results indicate that coated cathodes could produce not only safer Li-S batteries with long lifespans, but also efficient batteries with other metal sulfides, according to Wang’s team.
For more information, contact
Top Stories
NewsSensors/Data Acquisition
![]() Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
INSIDERRF & Microwave Electronics
![]() A Next Generation Helmet System for Navy Pilots
A Next Generation Helmet System for Navy Pilots
INSIDERWeapons Systems
![]() New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
NewsAutomotive
![]() Ford Announces 48-Volt Architecture for Future Electric Truck
Ford Announces 48-Volt Architecture for Future Electric Truck
INSIDERAerospace
![]() Active Strake System Cuts Cruise Drag, Boosts Flight Efficiency
Active Strake System Cuts Cruise Drag, Boosts Flight Efficiency
ArticlesTransportation
Webcasts
Aerospace
![]() Cooling a New Generation of Aerospace and Defense Embedded...
Cooling a New Generation of Aerospace and Defense Embedded...
Energy
![]() Battery Abuse Testing: Pushing to Failure
Battery Abuse Testing: Pushing to Failure
Power
![]() A FREE Two-Day Event Dedicated to Connected Mobility
A FREE Two-Day Event Dedicated to Connected Mobility
Automotive
![]() Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Electronics & Computers
![]() Advantages of Smart Power Distribution Unit Design for Automotive &...
Advantages of Smart Power Distribution Unit Design for Automotive &...
Unmanned Systems
![]() Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance
Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance