Towards Greater Sensitivity: A Brief FTIR and Infrared-Based Cavity Ring Down Spectroscopy Comparative Study

A threat in the form of chemical vapor may not be visible, but rapid detection is critical for preservation of life and property. In addition, understanding the surrounding environment informs the posture that the warfighter will need to take. The field of chemical vapor detection spans far beyond the warfighter and is rich in research. A search in SciFinder for “chemical vapor detection” provides over 400,000 results with over 3,000 books, 26,000 reviews, and nearly 300,000 journal articles. The focus of this document will be with an eye towards perimeter monitoring for a wide range of gas-phase chemicals. To accomplish such sensing, compound-specific sensors should not be employed as they lack capability to detect or inform about the presence of many potential threats outside of their selected targets. A viable technique for sensing a wide range of compounds is infrared absorption as most potential threats provide an infrared absorbance spectrum which arises from each compound’s unique molecular structure.
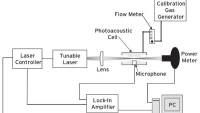
The purpose of this article is to provide a comparison between a commercially available instrument long utilized as a standard within several defense laboratories around the globe which employs FTIR methods for detection of environmental gasses in industrial environments to a newer class of IR absorption-based detectors that use cavity ringdown to determine the absorption profile.
While there are many ways to sense gases, optical, infrared absorption-based techniques offer the capability to perform real-time, in situ analysis and direct measurement of the vibrations of a molecule (in near- and mid-IR techniques). A keen advantage is gained when using infrared absorption techniques by applying a Fourier transform to the IR signal which allows for increased throughput (known as Jacquinot’s advantage) and multiplexing (known as Felleget’s advantage) and can increase the signal-to-noise.
Detailed information on the mechanism of IR absorption is extensively documented in scientific literature. In short, gas-phase IR analysis of a chemical provides vibration-rotation spectra. For a compound to be IR-active, there must be a change in the molecule’s dipole moment with respect to the molecular vibration; this is the basis of the absorption of the IR radiation and thereby a change in the spectral absorption profile. In addition to individual bonds within a molecule, there are also larger moieties within the molecule which vibrate at characteristic frequencies which provide structural or functional information and are nearly independent from the rest of the molecule’s structure.
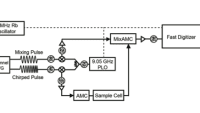
Cavity Ring-Down spectroscopy (CRDS) is a newer IR absorption-based technique made possible by the use of tunable pulsed lasers. Rather than using a broadband (blackbody) emitter as is done with traditional IR techniques, a pulsed laser is tuned across wavelengths into an optical cavity. Instead of measuring the absorption directly, the amount of light leaking through a highly reflective mirror within the cavity is measured with respect to time at each wavelength and a time constant for the decay can be measured.
This work was performed by Eric Languirand and Ian Pardoe for the U.S. Army Combat Capabilities Development Command (DEVCOM) Chemical Biological Center. For more information, download the Technical Support Package (free white paper) below. CBCTN-093
This Brief includes a Technical Support Package (TSP).

Towards Greater Sensitivity: A Brief FTIR and Infrared-Based Cavity Ring Down Spectroscopy Comparative Study
(reference CBCTN-093) is currently available for download from the TSP library.
Don't have an account?
Overview
The document titled "Towards Greater Sensitivity: A Brief FTIR and Infrared-Based Cavity Ring Down Spectroscopy Comparative Study" presents a comparative analysis of two advanced spectroscopic techniques: Fourier Transform Infrared (FTIR) spectroscopy and Infrared-Based Cavity Ring Down Spectroscopy (CRDS). Authored by Eric R. Languirand and Ian J. Pardoe from the U.S. Combat Capabilities Development Command Chemical Biological Center (DEVCOM CBC), the report was finalized in March 2023 and is publicly available.
The study aims to enhance the sensitivity of vapor detection methods, which is crucial for applications in chemical detection and environmental monitoring. The document begins with an overview of the two techniques, detailing their principles and operational mechanisms. FTIR is a well-established method that provides qualitative and quantitative analysis of gases, while CRDS is a newer technology that offers higher sensitivity by measuring the decay time of light in a cavity filled with the sample gas.
The report outlines the system descriptions for the Gasmet DX4000 and RingIR AG-4000 instruments used in the experiments. It describes the experimental setup, including vapor generation and cavity fill procedures, and details the test procedures employed to ensure accurate and reliable measurements. Key aspects of the testing include daily background measurements, concentration verification, and humidity control.
Results and observations from the experiments are discussed, highlighting the performance differences between FTIR and CRDS. The findings suggest that CRDS provides superior sensitivity, making it a more effective choice for detecting low concentrations of analytes in various environments. The report also addresses potential future work, indicating areas for further research and development to optimize these technologies for practical applications.
In conclusion, the document emphasizes the importance of advancing detection technologies in the context of chemical and biological threats. By comparing FTIR and CRDS, the study contributes valuable insights into the capabilities and limitations of each method, ultimately supporting the development of more sensitive and reliable detection systems for military and civilian use. The report is part of ongoing efforts to enhance the Army's capabilities in chemical detection and response.
Top Stories
NewsRF & Microwave Electronics
![]() Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
INSIDERAerospace
![]() A Next Generation Helmet System for Navy Pilots
A Next Generation Helmet System for Navy Pilots
INSIDERDesign
![]() New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
INSIDERMaterials
![]() How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
NewsPower
![]() Ford Announces 48-Volt Architecture for Future Electric Truck
Ford Announces 48-Volt Architecture for Future Electric Truck
ArticlesAR/AI
Webcasts
Electronics & Computers
![]() Cooling a New Generation of Aerospace and Defense Embedded...
Cooling a New Generation of Aerospace and Defense Embedded...
Automotive
![]() Battery Abuse Testing: Pushing to Failure
Battery Abuse Testing: Pushing to Failure
Power
![]() A FREE Two-Day Event Dedicated to Connected Mobility
A FREE Two-Day Event Dedicated to Connected Mobility
Unmanned Systems
![]() Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Automotive
![]() Advantages of Smart Power Distribution Unit Design for Automotive &...
Advantages of Smart Power Distribution Unit Design for Automotive &...
Energy
![]() Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance
Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance