Sodium-Ion Battery Anode for Energy Storage
Carbonaceous anodes based on organic pigments exhibit a high sodium-ion storage performance and excellent cycle stability.
Lithium-ion batteries have high energy density and a long cycle life, making them indispensable in portable electronics as well as electric vehicles. However, the high cost and limited supply of lithium necessitate the development of alternative energy storage systems. To this end, researchers have suggested sodium-ion batteries (SIBs) as a possible candidate.
Besides having physicochemical properties similar to that of lithium, sodium is both sustainable and cost-effective. However, its ions are large with sluggish diffusion kinetics, hindering their accommodation within the carbon microstructures of the commercialized graphite anodes. Consequently, SIB anodes suffer from structural instability and poor storage performance. In this regard, carbonaceous materials doped with heteroatoms are showing promise. However, their preparation is complicated, expensive, and time-consuming.
A team of researchers, led by Professor Seung Geol Lee from Pusan National University in Korea, used quinacridones as precursors to prepare carbonaceous SIB anodes. “Organic pigments such as quinacridones have a variety of structures and functional groups. As a result, they develop different thermal decomposition behaviors and microstructures. When used as a precursor for energy storage materials, pyrolyzed quinacridones can greatly vary the performance of secondary batteries. Therefore, it is possible to implement a highly efficient battery by controlling the structure of organic pigments precursor,” explained Lee.
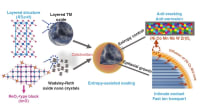
The researchers focused on 2,9-dimethylquinacridone (2,9-DMQA) in their study. 2,9-DMQA has a parallel molecular packing configuration. Upon pyrolysis (thermal decomposition) at 600 °C, 2,9-DMQA turned from reddish to black with a high char yield of 61 performed. The researchers next performed a comprehensive experimental analysis to describe the underlying pyrolysis mechanism.
They proposed that the decomposition of methyl substituents generates free radicals at 450 °C, which form polycyclic aromatic hydrocarbons with a longitudinally grown microstructure resulting from bond bridging along the parallel packing direction. Further, nitrogen- and oxygen-containing functional groups in 2,9-DMQA released gases, creating disordered domains in the microstructure. In contrast, pyrolyzed unsubstituted quinacridone developed highly aggregated structures. This suggested that the morphological development was significantly affected by the crystal orientation of the precursor.
In addition, 2,9-DMQA pyrolyzed at 600 °C exhibited a high rate capability (290 mAh/g at 0.05 A/g) and excellent cycle stability (134 mAh/g at 5 A/g for 1000 cycles) as an SIB anode. The nitrogen- and oxygen-containing groups further enhanced battery storage via surface confinement and interlayer distance increment.
“Organic pigments such as quinacridones can be used as anode materials in sodium-ion batteries. Given the high efficiency, they will provide an effective strategy for mass production of large-scale energy storage systems,” said Lee.
For more information, contact Professor Seung Geol Lee at
Top Stories
NewsRF & Microwave Electronics
![]() Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
INSIDERAerospace
![]() A Next Generation Helmet System for Navy Pilots
A Next Generation Helmet System for Navy Pilots
INSIDERDesign
![]() New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
INSIDERMaterials
![]() How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
NewsPower
![]() Ford Announces 48-Volt Architecture for Future Electric Truck
Ford Announces 48-Volt Architecture for Future Electric Truck
ArticlesAR/AI
Webcasts
Electronics & Computers
![]() Cooling a New Generation of Aerospace and Defense Embedded...
Cooling a New Generation of Aerospace and Defense Embedded...
Automotive
![]() Battery Abuse Testing: Pushing to Failure
Battery Abuse Testing: Pushing to Failure
Power
![]() A FREE Two-Day Event Dedicated to Connected Mobility
A FREE Two-Day Event Dedicated to Connected Mobility
Unmanned Systems
![]() Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Automotive
![]() Advantages of Smart Power Distribution Unit Design for Automotive &...
Advantages of Smart Power Distribution Unit Design for Automotive &...
Energy
![]() Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance
Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance