Designing Sodium-Ion Batteries with Extended Longevity
A whole new electrolyte recipe generates an ultra-thin protective layer that contributes to additional stability.

Cheap and abundant, sodium is a prime promising candidate for new battery technology. But limited performance of sodium-ion batteries has hindered their large-scale applications. Now, a research team from the Department of Energy’s Pacific Northwest National Laboratory has developed a sodium-ion battery with greatly extended longevity in laboratory tests.
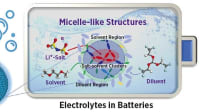
In batteries, electrolyte is the circulating “blood” that keeps the energy flowing. The electrolyte forms by dissolving salts in solvents, resulting in charged ions that flow between the positive and negative electrodes. Over time, the electrochemical reactions that keep the energy flowing get sluggish, and the battery can no longer recharge. In current sodium-ion battery technologies, this process happens much faster than in similar lithium-ion batteries.
The PNNL team attacked that problem by switching out the liquid solution and the type of salt flowing through it to create a wholly new electrolyte recipe. In laboratory tests, the new design proved durable, holding 90 percent of its cell capacity after 300 cycles at 4.2 V, which is higher than most sodium-ion batteries previously reported.
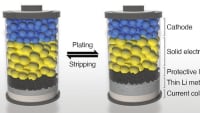
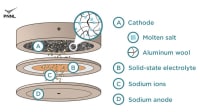
The current electrolyte recipe for sodium-ion batteries results in the protective film on the negative end (the anode) dissolving over time. This film is critical because it allows sodium ions to pass through while preserving battery life. The PNNL-designed technology works by stabilizing this protective film. The new electrolyte also generates an ultra-thin protective layer on the positive pole (the cathode) that contributes to additional stability of the entire unit.
The PNNL-developed sodium-ion technology uses a naturally fire-extinguishing solution that is also impervious to temperature changes and can operate at high voltages. One key to this feature is the ultra-thin protective layer that forms on the anode. This ultra-thin layer remains stable once formed, providing the long cycle life.
For now, the sodium-ion technology still lags behind lithium in energy density. But it has its own advantages, such as imperviousness to temperature changes, stability, and long cycle life, which are valuable for applications of certain light-duty electric vehicles and even grid energy storage in the future.
For more information, contact Karyn Hede at
Top Stories
NewsRF & Microwave Electronics
![]() Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
INSIDERAerospace
![]() A Next Generation Helmet System for Navy Pilots
A Next Generation Helmet System for Navy Pilots
INSIDERDesign
![]() New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
INSIDERMaterials
![]() How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
NewsPower
![]() Ford Announces 48-Volt Architecture for Future Electric Truck
Ford Announces 48-Volt Architecture for Future Electric Truck
ArticlesAR/AI
Webcasts
Electronics & Computers
![]() Cooling a New Generation of Aerospace and Defense Embedded...
Cooling a New Generation of Aerospace and Defense Embedded...
Automotive
![]() Battery Abuse Testing: Pushing to Failure
Battery Abuse Testing: Pushing to Failure
Power
![]() A FREE Two-Day Event Dedicated to Connected Mobility
A FREE Two-Day Event Dedicated to Connected Mobility
Unmanned Systems
![]() Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Automotive
![]() Advantages of Smart Power Distribution Unit Design for Automotive &...
Advantages of Smart Power Distribution Unit Design for Automotive &...
Energy
![]() Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance
Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance