Fuel-Cell Nanoparticle Clusters May Cut Platinum Catalyst Costs
The Technical University of Munich research team reveals findings on optimizing platinum clusters for fuel-cell catalysts.

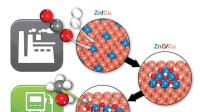
Fuel-cell technology is a big subject with some very small but vital aspects, one of which is the size of atomic platinum clusters. Reduce these and the amount of platinum needed (one of the major cost drawbacks of current fuel-cell systems) would also fall. An interdisciplinary research team at the Technical University of Munich (TUM) has just detailed in a report of successfully modeling size-optimized catalysts that are twice as good as the best pure platinum catalysts commercially available today.
“In fuel cells, hydrogen reacts with oxygen to create water, generating electricity in the process,” explained TUM’s Roland Fischer, professor of inforganic and organometallic chemistry. “Sophisticated catalysts at the electrodes are required in order to optimize this conversion and platinum plays a central role in the oxygen-reduction reaction.” The TUM team members were looking to answer how small a cluster of platinum atoms can be and still have a highly active catalytic effect.
Fischer said the ideal size for platinum stacks measure about one nanometer, containing some 40 platinum atoms. Fischer’s colleague, Aliaksandr Bandarenka professor of physics of energy conversion and storage, noted that, “platinum catalysts of this order of size have a small volume but a large number of active spots, resulting in high mass activity.” The team worked with the Catalysis Research Center at TUM to refine its results, combining theoretical capabilities in modeling, physics and chemistry with specialist capability to create and experimentally test the calculated platinum nanocatalyst.
Though the results are encouraging and strengthen fuel-cell cost equations, more research will be needed in order to build a commercial case. For commercial use, the 50% reduction in platinum based on the team’s findings would need to increase to 80%. Beyond the nanoparticles’ spherical shape, higher catalytic activity may be achieved via more complex configurations Bandarenka said, noting that more complex shapes require more complex synthesis methods.
Top Stories
NewsSensors/Data Acquisition
![]() Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
INSIDERRF & Microwave Electronics
![]() A Next Generation Helmet System for Navy Pilots
A Next Generation Helmet System for Navy Pilots
INSIDERWeapons Systems
![]() New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
NewsAutomotive
![]() Ford Announces 48-Volt Architecture for Future Electric Truck
Ford Announces 48-Volt Architecture for Future Electric Truck
INSIDERAerospace
![]() Active Strake System Cuts Cruise Drag, Boosts Flight Efficiency
Active Strake System Cuts Cruise Drag, Boosts Flight Efficiency
ArticlesTransportation
Webcasts
Aerospace
![]() Cooling a New Generation of Aerospace and Defense Embedded...
Cooling a New Generation of Aerospace and Defense Embedded...
Energy
![]() Battery Abuse Testing: Pushing to Failure
Battery Abuse Testing: Pushing to Failure
Power
![]() A FREE Two-Day Event Dedicated to Connected Mobility
A FREE Two-Day Event Dedicated to Connected Mobility
Automotive
![]() Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Electronics & Computers
![]() Advantages of Smart Power Distribution Unit Design for Automotive &...
Advantages of Smart Power Distribution Unit Design for Automotive &...
Unmanned Systems
![]() Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance
Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance