Portable Gas Detection Shrinks to New Dimensions

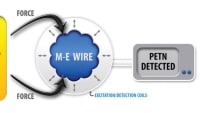
A sensor for detecting toxic gases is now smaller, faster and more reliable, setting it up for integration into a highly sensitive portable system for detecting chemical weapons. Better miniature sensors can also rapidly detect airborne toxins where they occur, providing key information to help emergency personnel respond safely and effectively to an incident.
Chemical identification typically involves collecting a sample at the scene of a chemical release and bringing it back to a room full of equipment operated by trained personnel. The machines sift through a sample of various gases and weigh the molecules to determine their identities. And while portable versions of these instruments, known as mass spectrometers, are commercially available, they are less sensitive than their lab-based counterparts.
For more than 20 years, researchers at Sandia National Laboratories have been working to avoid the performance penalty for portable gas detection. Their sensors employ a technique called gas chromatography, or GC for short. Briefcase-sized instruments from Sandia have sniffed for nerve and blister agents continuously for 22 months in the Boston subway without a false alarm. Sensors about the size of a AA battery can detect a compound in sweat that signals smuggled humans. Handheld gas sensor systems can also monitor crop health by identifying gases that plants release when stressed by drought or sickness.
Now, Joshua Whiting, an analytical chemist at Sandia, and his colleagues shrunk their sensor to about the size of a dollar bill while also increasing the performance of the sensor. The system now separates a gas sample twice — yet the entire analysis happens in less than 10 seconds. The extra separation step reduces interference from solvents, cleaners and diesel fuel that could also be in the air during a chemical weapons release. Less interference also means the signal for detected target compounds is more reliable.
The researchers recently used the sensor to identify each ingredient of a 29-compound mixture in seven seconds. The trick to that rapid analysis is a pressure valve in the sensor that controls how quickly gases flow through each separation step. Controlling this flow with pressure means the sensor uses less energy than similar temperature-controlled systems. The system also reliably detected compounds that simulate mustard gas and phosphonate-based nerve agents during 40 days of continuous operation.
Energy efficiency, combined with reliable detection in an increasingly small package, sets the researchers up for the next phase of the project: building a fully portable analytical system with integrated chemical separation, selective detection and computerized data analysis that performs as well as — or better than — lab-based equipment.
Top Stories
INSIDERManufacturing & Prototyping
![]() How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
INSIDERManned Systems
![]() FAA to Replace Aging Network of Ground-Based Radars
FAA to Replace Aging Network of Ground-Based Radars
NewsTransportation
![]() CES 2026: Bosch is Ready to Bring AI to Your (Likely ICE-powered) Vehicle
CES 2026: Bosch is Ready to Bring AI to Your (Likely ICE-powered) Vehicle
NewsSoftware
![]() Accelerating Down the Road to Autonomy
Accelerating Down the Road to Autonomy
EditorialDesign
![]() DarkSky One Wants to Make the World a Darker Place
DarkSky One Wants to Make the World a Darker Place
INSIDERMaterials
![]() Can This Self-Healing Composite Make Airplane and Spacecraft Components Last...
Can This Self-Healing Composite Make Airplane and Spacecraft Components Last...
Webcasts
Defense
![]() How Sift's Unified Observability Platform Accelerates Drone Innovation
How Sift's Unified Observability Platform Accelerates Drone Innovation
Automotive
![]() E/E Architecture Redefined: Building Smarter, Safer, and Scalable...
E/E Architecture Redefined: Building Smarter, Safer, and Scalable...
Power
![]() Hydrogen Engines Are Heating Up for Heavy Duty
Hydrogen Engines Are Heating Up for Heavy Duty
Electronics & Computers
![]() Advantages of Smart Power Distribution Unit Design for Automotive...
Advantages of Smart Power Distribution Unit Design for Automotive...
Unmanned Systems
![]() Quiet, Please: NVH Improvement Opportunities in the Early Design...
Quiet, Please: NVH Improvement Opportunities in the Early Design...