Oximeter Headband Initial Characterization Test on Form, Fit, and Function
Developing a real-time, nonintrusive blood oxygenation sensor system capable of monitoring physical parameters such as altitude acclimation and shock in the event of trauma or hemorrhage, as well as providing early detection of viremia.

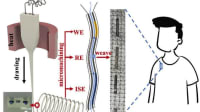
The oximeter headband is a new physiological status monitoring system developed by Massachusetts Institute of Technology Lincoln Laboratory (MIT LL) in Lexington, Massachusetts and the Institute for Advanced Functional Fabrics of America (AFFOA) in Cambridge, Massachusetts. It was tested for form, fit, and function during an internal test held at MIT LL.
The oximeter headband system consists of a custom-made, textile-based headband. It is a noninvasive, non-subcutaneous, and non-radiation-harming device that only maintains surface contact with the skin. It has been specifically designed to meet the needs of the military. The test utilized in this research assesses the first field-portable prototype of the oximeter headband device. Data was collected from eight test participants in two test scenarios, a functional evaluation, and a form and fit assessment.
Blood oxygenation (SpO2) is an important metric for monitoring altitude acclimation, monitoring shock in the event of trauma or hemorrhage, as well as early detection of viremia. An individual’s SpO2 can drop acutely in response to these environmental changes, often without warning. In these scenarios, continuous, real-time monitoring of blood oxygenation provides the ability for early warning and enables corrective action to take place prior to more adverse outcomes.
This type of real-time SpO2 monitoring is particularly beneficial in use cases where a single end-user is monitoring many individuals, such as a squad medic monitoring their entire team. However, commercial pulse oximeters currently available are either tethered to benchtop electronics, limited in their wear location, or only allow for a single patient or individual to be monitored by a single device.
Employing finger pulse oximeters or standard adhesive-type patches is not practical for many field applications because they interfere with the activities the user must accomplish, or they are impractical for other varying reasons. Specifically, the finger-worn SpO2 monitor requires the user to position the sensor on the finger and remain relatively stationary for the assessment to take place.
Conversely, there are monitoring systems primarily for use in the fitness arena that could be acceptable to wear, can be used in harsh environments, and do not interfere with job performance. These devices do not provide the accurate and precise physiological data needed to make mission or safety decisions, however. For example, the SpO2 monitor on the commercially available Apple Watch 6 (Apple Inc., Cupertino, CA) requires the user to remain motionless with their arm in a horizontal position for ten seconds. This is unacceptable for real-time monitoring of warfighters while undergoing training or actual missions.
A suitable system must produce valid data, work reliably in the specific environment for which it is intended, be comfortable and easy to use, and not inhibit motion or job performance. Furthermore, sensors must be of minimal weight and size, consume little power, and have negligible impact on the body. They must also provide accurate data that can be analyzed to provide actionable decision-making. To function in real time, the wearable SpO2 sensor must have reliable wireless body area network connections. Without connectivity, PSM use reverts to physiological data collection and storage for post hoc analysis.
Recently, a prototype oximeter headband system was developed to provide real time SpO2 monitoring for a team of individuals. The purpose of this present test was to assess the form, fit, and function of the oximeter headband prototype system. In addition to the functionality of the developed SpO2 sensor system, the human factors aspects of the system were also evaluated. This research documents the first assessment of the first set of field portable prototypes.
This work was performed by L. E. Cantley, D. C. Maurer, T. Wang, M. Ibanescu, and W. Tharion for the Massachusetts Institute of Technology Lincoln Laboratory. For more information, download the Technical Support Package (free white paper). MIT-0006
This Brief includes a Technical Support Package (TSP).

Oximeter Headband Initial Characterization Test on Form, Fit, and Function
(reference MIT-0006) is currently available for download from the TSP library.
Don't have an account?
Overview
The document is a technical report (TR-1268) produced by MIT Lincoln Laboratory, detailing the initial characterization tests of an oximeter headband designed for non-invasive physiological monitoring. The report is dated October 14, 2021, and is based on work supported by the Department of the Army under Air Force Contract No. FA8702-15-D-0001.
The primary focus of the report is to evaluate the form, fit, and function of the oximeter headband, which is intended to provide accurate and reliable measurements of physiological parameters, such as blood oxygen levels, in a comfortable and user-friendly manner. The authors of the report include L.E. Cantley, D.C. Maurer, T. Wang, M. Ibanescu, and W. Tharion, who conducted the studies at Lincoln Laboratory, a federally funded research and development center.
The report outlines the methodology used in the characterization tests, including the design considerations for the headband, the testing environment, and the parameters measured during the evaluation. It emphasizes the importance of ensuring that the device fits well and functions effectively in various conditions, which is crucial for its intended use in monitoring health metrics in real-time.
Additionally, the report includes a distribution statement indicating that it has been approved for public release, allowing for broad dissemination of the findings. The document also contains references to relevant regulations and guidelines regarding the use and distribution of the research material.
Overall, the report serves as a comprehensive overview of the initial testing phase of the oximeter headband, highlighting its potential applications in health monitoring and the collaborative efforts of the research team. It reflects the ongoing commitment to advancing technology in the field of physiological monitoring, with implications for both military and civilian health applications. The findings and recommendations presented in the report aim to inform future developments and improvements in the design and functionality of wearable health monitoring devices.
Top Stories
NewsAutomotive
![]() Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
Microvision Aquires Luminar, Plans Relationship Restoration, Multi-industry Push
INSIDERManned Systems
![]() A Next Generation Helmet System for Navy Pilots
A Next Generation Helmet System for Navy Pilots
INSIDERManufacturing & Prototyping
![]() New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
New Raytheon and Lockheed Martin Agreements Expand Missile Defense Production
NewsAutomotive
![]() Ford Announces 48-Volt Architecture for Future Electric Truck
Ford Announces 48-Volt Architecture for Future Electric Truck
INSIDERAerospace
![]() Active Strake System Cuts Cruise Drag, Boosts Flight Efficiency
Active Strake System Cuts Cruise Drag, Boosts Flight Efficiency
ArticlesAR/AI
Webcasts
Electronics & Computers
![]() Cooling a New Generation of Aerospace and Defense Embedded...
Cooling a New Generation of Aerospace and Defense Embedded...
Power
![]() Battery Abuse Testing: Pushing to Failure
Battery Abuse Testing: Pushing to Failure
Communications
![]() A FREE Two-Day Event Dedicated to Connected Mobility
A FREE Two-Day Event Dedicated to Connected Mobility
Transportation
![]() Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Quiet, Please: NVH Improvement Opportunities in the Early Design Cycle
Transportation
![]() Advantages of Smart Power Distribution Unit Design for Automotive &...
Advantages of Smart Power Distribution Unit Design for Automotive &...
Unmanned Systems
![]() Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance
Sesame Solar's Nanogrid Tech Promises Major Gains in Drone Endurance