Advanced Sensors for Traumatic Brain Injury (TBI)
Implanted sensors could be used to measure intracranial pressure in TBI patients.
The objective of this work was to use miniaturized, state-of-the-art pressure/temperature sensors engineered at Lawrence Livermore National Laboratory (LLNL) to measure the immediate increases in intracranial pressure (ICP) combined with longer-term measurements of biological ICP and intracranial temperature. The experience gathered from this work provided valuable data on sensor placement, long-term brain tissue responses to implanted sensors, and sensor capability of dual measurement of biologic ICP and impact pressure transients.
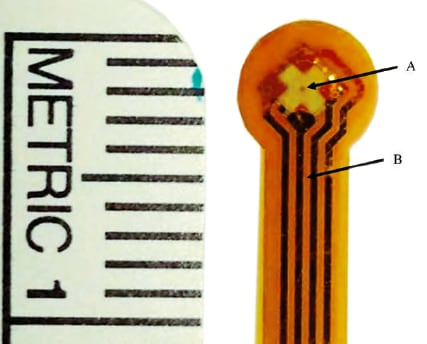
Test-ready sensors were produced with a range of diaphragm diameters (200 - 1000 μm). Diaphragm diameter should affect sensitivity of the sensors. These wafer-scale, absolute pressure sensors have an ultra-thin form factor (thickness 90 μm prior to packaging, and 130 μm after final packaging). The new sensors were designed to measure absolute pressure by modifying the original contact stress sensor design to create a reference cavity – a trapped volume of gas that is hermetically sealed within the device. The new sensors predefine the pressure sensor’s reference cavity within the silicon-on-insulator (SOI) wafer.
Five new sensors with diaphragm diameters of 200, 400, 600, 800, and 1000 μm (see figure) underwent testing and calibration to determine the optimum diaphragm diameter for subsequent inanimal testing. The new sensors were packaged with an encapsulating layer of Kapton to protect the sensor diaphragm and electrical connections from body fluids.
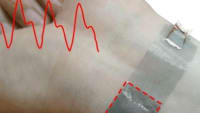
The first closed-diaphragm wafer sensors were received from LLNL and immediately tested. Static calibration produced a linear relationship between 5 and 25 PSI with an R2 = 0.986. The closed-diaphragm sensor is 90 microns thick, and the final product with Kapton packaging is 130 microns thick. Dynamic comparison of the new sensor with the existing fluid percussion pressure transducer provided close tracking of pressure events. Initial testing revealed the need for shielding of cables and power supply for subsequent applications.
This work was done by Bruce Lyeth, Ph.D., of the Regents of the University of California, Davis, for the Army Medical Research and Materiel Command. ARL-0190
This Brief includes a Technical Support Package (TSP).

Advanced Sensors for TBI
(reference AFRL-0190) is currently available for download from the TSP library.
Don't have an account?
Overview
The document is an annual report on the project titled "Advanced Sensors for TBI," led by Dr. Bruce Lyeth at the University of California, Davis, under the auspices of the U.S. Army Medical Research and Materiel Command. The report covers the period from July 1, 2014, to June 30, 2015, and outlines the project's objectives, accomplishments, and future plans.
The primary goal of the research was to develop new sensing technologies capable of measuring intracranial pressure (ICP) and temperature in real-time, particularly in the context of traumatic brain injury (TBI) models. The project aimed to utilize miniaturized, state-of-the-art pressure and temperature sensors engineered at Lawrence Livermore National Laboratory (LLNL). These sensors were designed to measure immediate increases in ICP and to provide long-term monitoring of biological ICP and intracranial temperature.
During the reporting period, the project achieved significant milestones, including the static calibration of new 1000 μm diaphragm sensors, which demonstrated a linear relationship between 5 and 25 PSI with a high correlation coefficient (R² = 0.986). Additionally, dynamic comparisons between the new sensors and existing fluid percussion pressure transducers showed close tracking of pressure events, indicating the sensors' reliability and accuracy.
The report notes that there were no training or professional development opportunities reported, nor were there any significant impacts on the principal disciplines, technology transfer, or society beyond science and technology. However, the project is positioned to transition into in situ animal testing, focusing on optimizing sensor sensitivity and range. Future plans include refining the sensors into a smaller package and improving the user interface for connecting the sensors to recording instruments. The team also intends to collaborate with a private company interested in commercializing the technology for TBI and other applications.
Overall, the report highlights the project's commitment to advancing sensor technology for TBI research, with a focus on preparing for testing in blast TBI models. The findings and developments from this project are expected to contribute valuable data for future studies and potential clinical applications.
Top Stories
INSIDERManufacturing & Prototyping
![]() How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
How Airbus is Using w-DED to 3D Print Larger Titanium Airplane Parts
INSIDERManned Systems
![]() FAA to Replace Aging Network of Ground-Based Radars
FAA to Replace Aging Network of Ground-Based Radars
NewsTransportation
![]() CES 2026: Bosch is Ready to Bring AI to Your (Likely ICE-powered) Vehicle
CES 2026: Bosch is Ready to Bring AI to Your (Likely ICE-powered) Vehicle
NewsSoftware
![]() Accelerating Down the Road to Autonomy
Accelerating Down the Road to Autonomy
EditorialDesign
![]() DarkSky One Wants to Make the World a Darker Place
DarkSky One Wants to Make the World a Darker Place
INSIDERMaterials
![]() Can This Self-Healing Composite Make Airplane and Spacecraft Components Last...
Can This Self-Healing Composite Make Airplane and Spacecraft Components Last...
Webcasts
Defense
![]() How Sift's Unified Observability Platform Accelerates Drone Innovation
How Sift's Unified Observability Platform Accelerates Drone Innovation
Automotive
![]() E/E Architecture Redefined: Building Smarter, Safer, and Scalable...
E/E Architecture Redefined: Building Smarter, Safer, and Scalable...
Power
![]() Hydrogen Engines Are Heating Up for Heavy Duty
Hydrogen Engines Are Heating Up for Heavy Duty
Electronics & Computers
![]() Advantages of Smart Power Distribution Unit Design for Automotive...
Advantages of Smart Power Distribution Unit Design for Automotive...
Unmanned Systems
![]() Quiet, Please: NVH Improvement Opportunities in the Early Design...
Quiet, Please: NVH Improvement Opportunities in the Early Design...